Discover the effect of PFAS regulations on the medical device industry, and explore our experts' 5 strategies to help medical device companies adapt to this evolving landscape.

Overcoming Wet Injection Challenges with Human-Centric Design
Although often overlooked, wet injections are a common usability issue in medical devices. Get the details on wet injections, and how we're using human-centric design to solve for them.
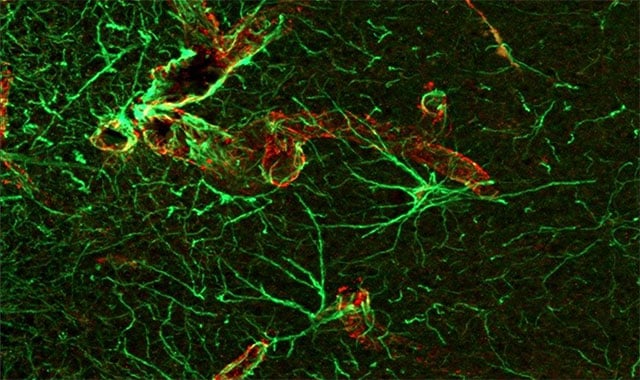
Stopping Brain Injury Before It Starts
Battelle received a five-year, $22 million contract through Defense Advanced Research Projects Agency’s Cornerstone program to create a preventative medical intervention to stop traumatic brain injury.
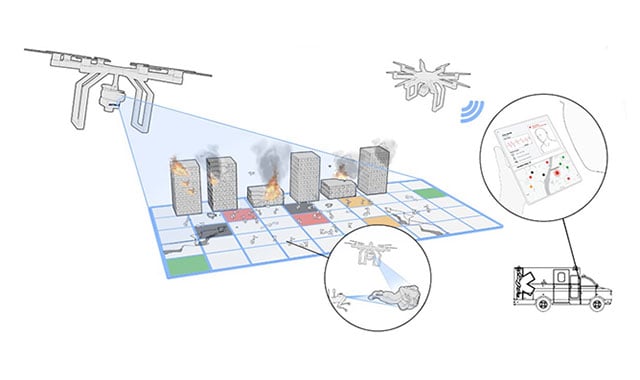
Tackling the Challenge of Mass Casualty Triage with Technology
Mass casualty incidents can occur anytime and anywhere, making it difficult for first responders to provide immediate assistance. Learn how we're using autonomous sensing and data fusion to solve for effective mass casualty triage.
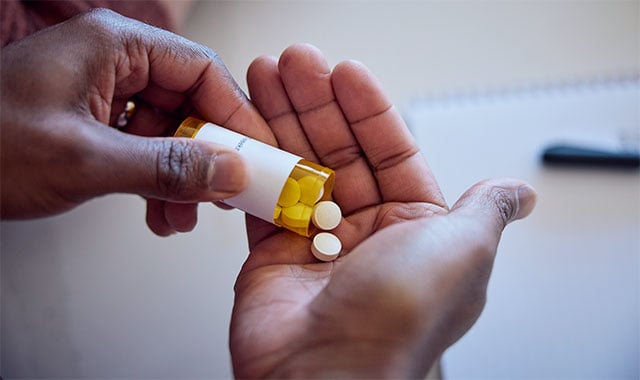
A Custom Pill for Every Patient: Transforming Personalized Medicine with 3D Printing
For patients that require multiple medications, adhering to complicated dosage schedules can be difficult. But what if multiple medications could be combined in one specialized pill?

Bridging Human Expertise and AI: The Future Is Here
The possibilities continue to expand for the future of human-AI teaming. Here's what Battelle Technical Fellow Justin Sanchez had to say about what's next for healthcare and AI.
Sign Up for Battelle Updates
Follow along with the latest news, announcement and updates from our Battelle community of solvers.
